|
SCIENTIFIC RESEARCH:
Spruce resin salve (ointment) made made
from Norway spruce (Picea abies) or other spruce species is
used for centuries around the world as part of traditional
folk medicine for treating infected wounds and ulcers. Lapp
people from northern Finland are one of the most known
society for using spruce resin remedies nowadays [1].
Medical doctors and scientists under the
supervision of dr. Arno Sipponen made extensive research
about healing properties of spruce salve (prepared
traditionally) in series of different experiments [2].
They were interested mainly on fact if beneficial properties
of spruce resin are just rumors or truth. Interest for
research on this field was raised due to the facts that
classical treatment methods such as cellulose polymer gauzes
are often unsuccessful and expensive [3].
|
Novel research which included
usage of spruce resin salve on patients in 11
different medical health centres in Finland
confirmed its significant role in faster recovery of
skin wounds and pressure ulcers [4-5].
Approximately 40 patients were randomly chosen for
therapy with traditionally prepared spruce resin
salve (resin group) or cellulose polymer
gauzes (sodium
carboxymethylcellulose hydrocolloid polymer)
(control group). Pressure ulcers are areas of skin
with superficial or deep tissue damage caused by
pressure, shear, friction or a combination of these.
It is more common with the people with limited
mobility (diseases or old age). Pressure ulcers are
difficult to treat, and there is, as yet, no ‘gold
standard’ for their treatment. The inclusion
criterion for research was grade II–IV pressure
ulcer. Exclusion criteria were a life expectancy of
less than 6 months or a malignant disease. The
primary outcome measure was complete healing of the
ulcer within 6 months. Secondary outcome measures
were partial healing of the ulcer, and successful
eradication of bacterial strains cultured from the
ulcers at study entry. All ulcers healed in 94% in
the resin group and in 44% patients in the control
group. During the 6-month therapy period, only 6% of
all ulcers was not healed in the resin group,
although there was much improvement.
Correspondingly, in the control treatment group 44%
of all ulcers were healed and 9% of ulcers in
the control group became even worse during the
follow-up. |
|
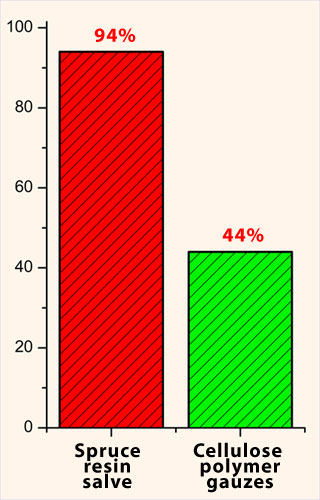
Comparison of
pressure ulcer healing between resin salve group
patients and cellulose polymer gauzes group
patients. |
| |
|
|
|
Traditional resin salve is significantly more
effective in the treatment of infected and
non-infected severe pressure ulcers than cellulose
polymer gauzes. |
| |
|
|
|
Spruce resin antimicrobial (and
antifungal) properties were studied against certain human
bacteria important in infected skin wounds [6-7].
Resin excretion obviously provides trees with protection
against bacterial and fungal infections. The resin salve
exhibited a bacteriostatic effect against all tested
Gram-positive bacteria but only against Proteus vulgaris
of the Gram-negative bacteria. Interestingly, the resin
inhibited the growth of bacteria, including methicillin-resistant
Staphylococcus aureus (MRSA) and vancomycin-resistant
Enterococcus (VRE), both on agar plates and in
culture media. The resin itself did not show any growth of
bacteria or fungi when tested in various growth media (blood
agar, chocolate agar, Sabouraud-dextrose agar, FAB medium).
Nor was there any microbial growth when resin had been
stored in the refrigerator or at room temperature for more
than 2 years.
|
|
SEM image of
MRSA. |
| |
|
The study directly confirmed
antimicrobial activity of the resin salve and
provided objective evidence of its antimicrobial
properties. The scientists also believe that spruce
resin take an active part in skin regeneration with
its compounds. It gives some explanations why the
traditional use of resin salve is experienced as
being effective in the treatment of infected skin
ulcers and healing of other similar skin injuries. |
| |
Literature:
 1. A Sipponen, et al., Drug Metabolism Letters, 2007, I; 143–145
1. A Sipponen, et al., Drug Metabolism Letters, 2007, I; 143–145
 2. J. Lohi, et al., Haava, 2006, 3; 10–13
2. J. Lohi, et al., Haava, 2006, 3; 10–13
 3. E. Eriksson, et al., Finnish Medical Journal, 1999, 54;
921–925
3. E. Eriksson, et al., Finnish Medical Journal, 1999, 54;
921–925
 4. A. Sipponen, Journal of Wound Care, 2007, 16; 72–74
4. A. Sipponen, Journal of Wound Care, 2007, 16; 72–74
 5. A. Sipponen, et al., British Journal of Dermatology,
2008, 158; 1055–1062
5. A. Sipponen, et al., British Journal of Dermatology,
2008, 158; 1055–1062
 6. J.L. Rios, et al., Journal of Ethnopharmacology, 2005,
51; 80–84
6. J.L. Rios, et al., Journal of Ethnopharmacology, 2005,
51; 80–84
 7. M. Rautio, et al., APMIS, 2007, 115; 335–340
7. M. Rautio, et al., APMIS, 2007, 115; 335–340 |